
The global prevalence of type 1 diabetes (T1D) continues to climb, with cases projected to nearly double from 8.4 million to nearly 17 million by 20401. Yet as the numbers rise, so too does the pace of innovation.
We’ve previously talked about the evolving field of diabetes research, exploring how advances in stem cell transplantation and emerging gene and immune-based therapies are inching us closer to insulin independence. On World Diabetes Day 2025, however, we wanted to take a closer look at the most cutting-edge technologies, both highlighting those helping ease the daily management burden, as well as providing an update on the ongoing efforts to find a scalable, durable cure.
Engineered Stem Cells Lead the Charge
Stem cell-derived treatments for T1D have continued to rapidly progress, with increasingly more approaches being trialled to overcome the same fundamental challenge: protecting transplanted β cells from both alloimmunity (immune rejection of donor cells) and autoimmunity (the underlying immune attack that causes T1D in the first place).
Vertex Pharmaceuticals’ zimislecel (formerly VX-880), an allogeneic (donor-derived) stem cell islet transplantation therapy, represents the most advanced approach to date. Recent positive data from the Phase 1/2 portion of the pivotal FORWARD-101 trial demonstrated that all twelve participants regained endogenous insulin secretion, with ten becoming fully insulin-independent one year after infusion2. The programme is now progressing into its phase 3 portion, with global regulatory submissions anticipated in 2026.
The holy grail, however, remains a therapy that eliminates the need for concurrent immunosuppression altogether. This is where newer gene-edited, “immune evasive” cell platforms are beginning to make headway. CRISPR Therapeutics’ VCTX210 and Sana Biotechnology’s UP421 both use advanced genome editing to create cells designed to avoid immune recognition. These “hypoimmune” (HIP) cells are genetically modified to delete or suppress key HLA class I and II molecules (which normally signal ‘foreign’ to the immune system), as well as overexpress protective markers such as CD47 to reduce recognition by natural killer immune cells. Notably, Sana’s UP421 recently achieved a world-first: insulin production from allogeneic islets in a fully immunocompetent patient without immunosuppressants, with sustained C-peptide (a key marker of endogenous insulin) production at 6 months indicating long-term graft survival — a landmark step towards functional immune evasion.
Meanwhile, autologous approaches, which sidestep alloimmunity altogether, are also showing early promise. A first-in-human Phase I trial in China demonstrated that patient-derived ‘CiPSC-islets’, reprogrammed from a patient’s own fat cells, could restore complete insulin independence within 75 days. One year later, time-in-range glucose levels exceeded 98% and no transplant-related complications were observed3.
Holding back the Onset: Advances in Early Intervention
While cell therapies aim to restore insulin, another front in diabetes innovation is focussed on disease prevention. By catching immune-driven β-cell loss early, researchers hope to delay, or even halt, the onset of clinical T1D.
One of the most significant breakthroughs in early intervention has been Teplizumab (Tzield®). This humanised anti-CD3 monoclonal antibody acts by modulating CD4+ and CD8+ T cells, key players in the immune attack on pancreatic β cells. Phase 3 clinical trials involving 217 patients have shown that a single course of treatment can result in significantly higher stimulated C-peptide levels at week 78 compared with those receiving placebo4. In August 2025, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) approved Tzield for use in adults with Stage 2 T1D, marking it as the country’s first-ever licensed immunotherapy for T1D and signalling a broader shift from treating established disease toward early immune intervention in at-risk individuals.
A range of other immunomodulatory therapies are also being explored with the aim of preventing or delaying islet autoantibodies and the loss of β-cell function. These include Abatacept, a CTLA-4 immunoglobulin that inhibits T-cell costimulatory signalling through the CD80–CD86 pathway, as well as cytokine modulators and JAK inhibitors, which act to dampen inflammatory pathways. Forthcoming clinical trial results will be critical in determining their potential role in early intervention.
Medtech and the Future of Diabetes Management
The burden of managing T1D continues to be alleviated by ongoing progress in Medtech. Implantable continuous glucose monitors (CGMs), such as Glucotrack, promise direct blood sampling, eliminating the typical lag time associated with using external sensors. Initial human trials reported a MARD (a measure of CGM accuracy) of 7.7% across 122 matched pairs alongside near‑complete data capture, demonstrating both excellent accuracy and reliability. A follow-up long-term feasibility study is expected to begin very soon. Given their lifespan of up to 3 years, such devices are ultimately paving the way towards a more reliable, lower-maintenance glucose monitoring solution.
Automated insulin delivery (AID) systems (Figure 1) are also transforming diabetes5. These devices combine insulin pumps, CGMs, and adaptive algorithms to automatically adjust insulin dosing based on real-time data. “Hybrid” closed-loop systems (which still require user-initiated meal boluses) already deliver notable improvements in blood glucose control, but a fully closed-loop “artificial pancreas” capable of predicting glucose fluctuations from meals, exercise, or stress without user input system is the ultimate goal. Although automated systems that include real-time meal detection algorithms are now entering clinical trials with promising results6, many challenges remain, including fine-tuning the algorithm so it can adapt to the rapidly changing insulin requirements within an individual.
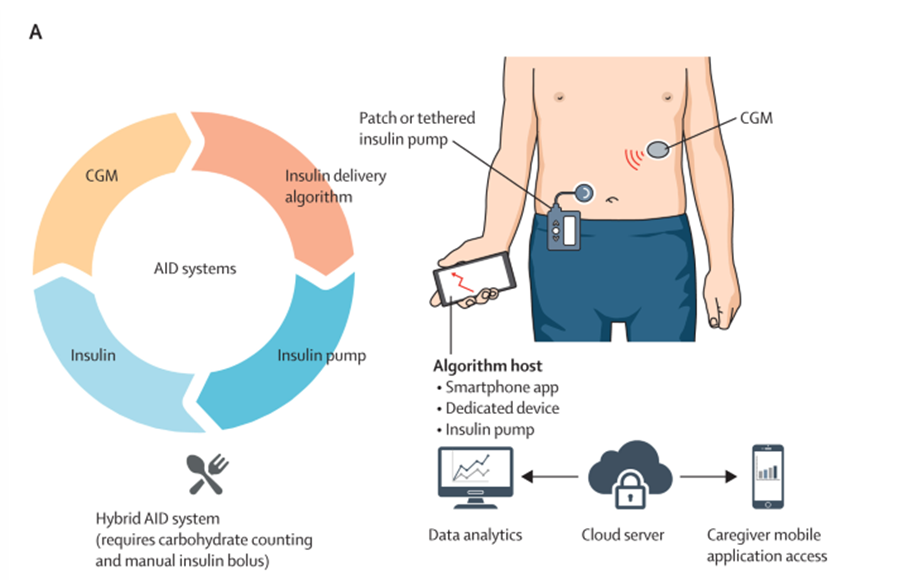
Figure 1 - Automated insulin delivery (AID) systems and their connected components.
Adapted from Ziegler et al., 2025.5
Intelligent Innovation: What’s Coming Next?
Other innovative approaches to a T1D cure are starting to enter the space. Sonoma Biotherapeutics is advancing regulatory T-cell (Treg) therapies, which are designed to restore immune tolerance by selectively suppressing autoimmunity. Early results from the REGULATE-RA Phase 1 trial on their lead CAR-Treg therapy show promising safety and efficacy in patients with refractory rheumatoid arthritis; proof of concept that the approach can work. With the growing momentum behind Treg therapies following the recent Nobel Prize recognition, Treg-based platforms could become a cornerstone of treatment for further autoimmune diseases, particularly if such early clinical success translates across indications.
Independently of this, research published earlier this month explored an entirely different route. Researchers showed that embryonic stem cell-derived gastric organoids can be reprogrammed into insulin-producing cells in vivo by introducing key pancreatic transcription factors. When transplanted into diabetic mice, these engineered organoids generated functional β-like cells that secreted insulin and normalised blood glucose levels7. While further work is needed to confirm long-term function and to fully replicate natural islet architecture, this approach highlights a novel, potentially autologous (and therefore immunosuppression-independent) avenue for β-cell replacement that could bypass donor shortages.
Like so many fields, diabetes care is also being reshaped by advances in artificial intelligence (AI), driving a shift towards more personalised and predictive management. The wealth of data generated by CGMs provides a rich resource for training machine-learning (ML) models, which are now being used to detect the earliest autoimmune changes in T1D. At the 2025 ADA Scientific Sessions, one abstract reported age-specific AI/ML models achieving 80% sensitivity in individuals under 25, identifying high-risk patients up to 12 months before the onset of stage 3 diabetes8. Similarly, trials such as on the ‘robust artificial pancreas (RAP)’ system have demonstrated that neural network models can automatically detect meals with 83.3% sensitivity and deliver recommended insulin doses, reducing time above range (glucose >180 mg/dL) by 10.8% compared to existing algorithms9. Whilst the potential of continuously-learning AI systems trained on real-world, multi-modal dataset is enormous, the field is in its infancy, meaning rigorous validation continues to be required to ensure they deliver safe, reliable guidance.
Outlook and Perspectives
The continued momentum in stem cell research means a definitive cure for T1D is closer than ever. However, simultaneous advances across Medtech and predictive AI are providing increasingly effective ways to manage the disease. Even though insulin is still required, tools such as automated insulin delivery, implantable CGMs, and personalised algorithms can significantly reduce the day-to-day burden. The coming decade will therefore likely see a convergence of strategies working in parallel: preventing or delaying onset in at-risk individuals, easing the burden for those currently living with the condition, and ultimately, developing a scalable, durable cure.
References:
- Gregory, G. A. et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 10, 741–760 (2022).
- Reichman, T. W. et al. Stem Cell–Derived, Fully Differentiated Islets for Type 1 Diabetes. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2506549 (2025) doi:10.1056/NEJMoa2506549.
- Wang, S. et al. Transplantation of chemically induced pluripotent stem-cell-derived islets under abdominal anterior rectus sheath in a type 1 diabetes patient. Cell 187, 6152-6164.e18 (2024).
- Ramos, E. L. et al. Teplizumab and β-Cell Function in Newly Diagnosed Type 1 Diabetes. N. Engl. J. Med. 389, 2151–2161 (2023).
- Ziegler, A.-G., Cengiz, E. & Kay, T. W. H. The future of type 1 diabetes therapy. The Lancet 406, 1520–1534 (2025).
- Garcia-Tirado, J. et al. Advanced Closed-Loop Control System Improves Postprandial Glycemic Control Compared With a Hybrid Closed-Loop System Following Unannounced Meal. Diabetes Care dc210932 (2021) doi:10.2337/dc21-0932.
- Lu, J. et al. Modeling in vivo induction of gastric insulin-secreting cells using transplanted human stomach organoids. Stem Cell Rep. 0, (2025).
- LAM, F. et al. 2058-LB: Identification of Earlier Stage Autoimmune Type 1 Diabetes Using Machine Learning Algorithms. Diabetes 74, 2058-LB (2025).
- Mosquera-Lopez, C. et al. Enabling fully automated insulin delivery through meal detection and size estimation using Artificial Intelligence. Npj Digit. Med. 6, 39 (2023).
Max is a patent technical assistant working in the Life Sciences team. Max has a BA in Cell and Systems Biology from the University of Oxford where he was awarded the Gibbs prize for graduating with best overall performance. He also holds an MSc in Bioinformatics from the University of Edinburgh, during which his final project focused on developing Python-based approaches to quantify alternative splicing events in innate immune genes.
Email: max.falk@mewburn.com
Sign up to our newsletter: Forward - news, insights and features
Our people
Our IP specialists work at all stage of the IP life cycle and provide strategic advice about patent, trade mark and registered designs, as well as any IP-related disputes and legal and commercial requirements.
Our peopleContact Us
We have an easily-accessible office in central London, as well as a number of regional offices throughout the UK and an office in Munich, Germany. We’d love to hear from you, so please get in touch.
Get in touch
.png)
