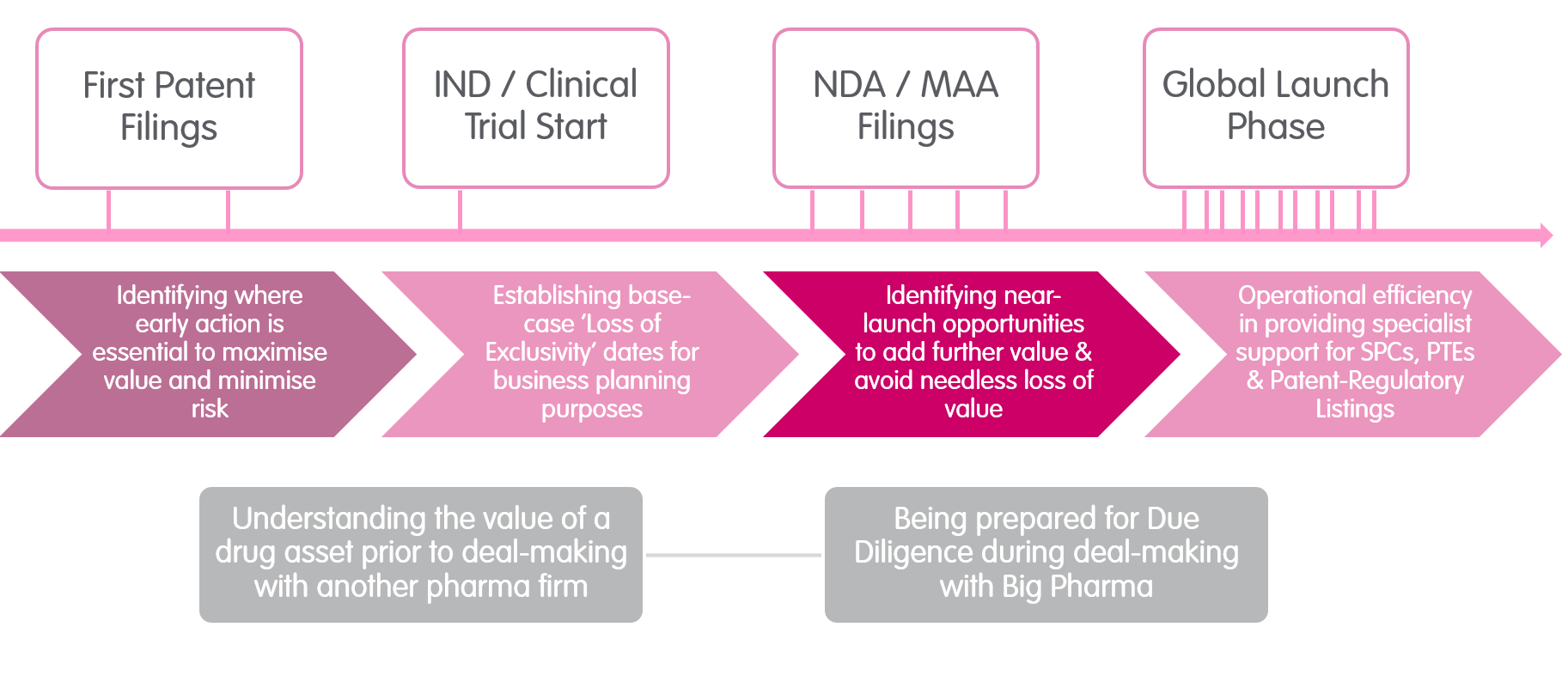
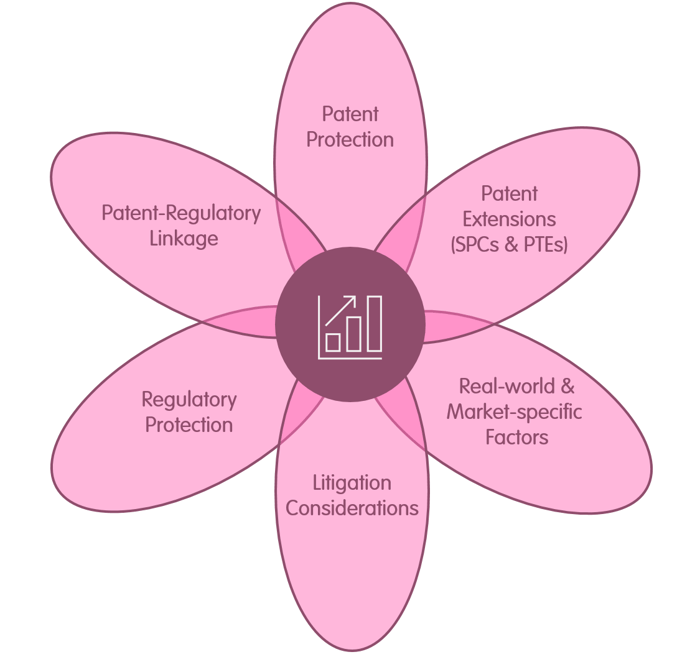
Our Speciality Pharma Team provides a rare fusion of six critical areas for any drug product, delivering valuable insights and strategies to add value, mitigate risk, avoid needless loss of value and help deliver commercial clarity for a given drug. While some law firms may have expertise in two or three of the key areas identified below, our team pulls together all six in one group.
Unusually, our team draws on insights from over 10 years’ in-house IP experience at a global biopharmaceutical firm. This enhances our knowledge and expertise to provide such specialist support not just for Europe, but on a worldwide basis. Having such multi-jurisdictional capability concentrated in one team is incredibly valuable for clients who are looking for a global analysis for their drug asset. From an operational perspective, not only can our expertise bring efficiencies and consistency when handling SPC/PTE filings, but there are cross-over efficiencies available where we also support Patent-Regulatory Listings at the same time. With so many valuable drug products starting life in a small pharma firm, we are well equipped to support our clients through the partnering and due diligence phases that are often part of the drug’s overall journey to the global market.
.png)