Bespoke cell therapies are expensive and time-consuming. Now allogeneic cell treatments offer a rapid off-the-shelf alternative.
Forward: features are independent pieces written for Mewburn Ellis discussing and celebrating the best of innovation and exploration from the scientific and entrepreneurial worlds.
Thirteen-year-old Alyssa had exhausted all conventional treatments and was facing palliative care for her leukaemia when she became the first person in the world to receive a pioneering cell therapy.
The cutting-edge treatment cleared the cancer from her body, leaving her family on cloud nine and paving the way for other, similar treatments and better futures for cancer patients around the world.
Alyssa was given a CAR T-cell therapy developed at Great Ormond Street Hospital and UCL that was composed of immune cells that had been genetically engineered to seek out and destroy her cancer.
Excitingly, and in a crucial development over all the CAR T-cell therapies that are currently available, Alyssa’s therapy was an allogeneic treatment, meaning that she received immune cells from a healthy donor, rather than being given her own modified immune cells.
Cells tailored to each patient
CAR T-cell therapies have already produced unprecedented results in the treatment of cancer, with reports of patients who had run out of options and had just months – or weeks – to live going into remission, or even being cured, by a single dose.
Six CAR T-cell therapies have been approved to treat blood cancers in Europe and the US since 2017. They are evolving from being a last-resort treatment to being given earlier in the course of the cancer, when they may be even more effective.
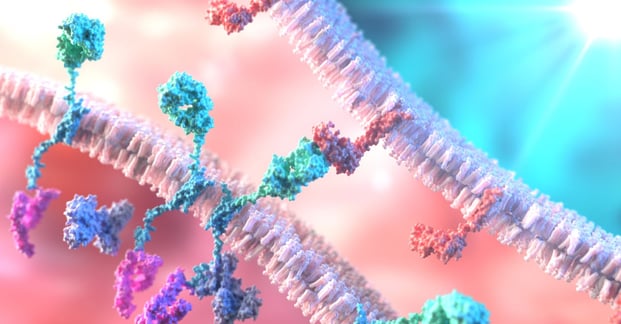
T-Cell binding to a tumour cell using Chimeric Antigen Receptor (CAR)
The true potential of the CAR-T revolution is, however, being stymied by two key problems – the cost and the length of time it takes to produce each CAR T-cell therapy. Currently-approved CAR-T therapies are autologous and produced from immune cells obtained from each patient. Each individual CAR T-cell therapy is therefore personalised to a specific patient. The patient’s own blood is drawn and their T-cells isolated and genetically engineered to produce chimeric antigen receptors (CARs); proteins on the surface of the T-cells that recognise and target specific molecules on the surface of cancer cells.
Multiplied in the lab and reinfused into the patient in their hundreds of millions, the T-cells home in on and kill cancer cells bearing the specific target molecule. The ‘living drug’ then lingers in the body to stop the cancer from returning.
However, while CAR T-cell therapies can mean the difference between life and death for some patients, autologous therapies come with a price tag of hundreds of thousands of pounds per patient and take around a month to make. ‘The costs are unbelievably high,’ says Dr John Connolly, CSO at the Parker Institute for Cancer Immunotherapy in San Francisco. ‘It’s like if every time you took aspirin, I had to manufacture that aspirin just for you. It’s difficult to find ways to push the cost down.
‘The other issue is that because I'm manufacturing it for every patient, there's a lead time. There are plenty of patients where we’ve started the manufacturing process and they’ve progressed way too far by the time that it was ready. So they never received the drug, or by the time they received it, they were just absolutely overwhelmed with cancer.’
From tailor-made to off-the-shelf
Many believe that the solution to these problems is allogeneic therapies, such as the treatment given to Alyssa. Rather than being made on a bespoke basis, allogeneic therapies are off-the-shelf treatments that can be manufactured at scale from donor cells and then kept on ice, ready for use.
More affordable and more accessible, allogeneic CAR-T therapies may also be more powerful, as the T-cells at their core come from healthy donors, rather than from a patient whose immune cells may already have been buffeted by multiple rounds of chemotherapy and radiotherapy. As the saying goes in medicine: ‘the best donor is a healthy donor’.
‘Here you have a very convenient treatment,’ says Dr Ivan Horak, CSO and CMO at Singapore-based Tessa Therapeutics, a cancer immunotherapy biotech with a focus on allogeneic therapies. ‘The quality of the cells is very predictable and the manufacturing process is faster and cheaper.’
Horak has good reason to be excited. His company, working with Baylor College of Medicine, has recently released compelling data on its off-the-shelf CAR T-cell therapy TT11X in patients with Hodgkin’s lymphoma. Three-quarters of the 14 patients in a phase 1 trial responded to TT11X and half achieved complete remission, meaning there was no longer any sign of their disease on scans.
%20bind%20to%20CD19%20molecules%20on%20a%20leukemia%20cell%20leading%20to%20segregation%20of%20granzyme%20vesicles%20(yellow)%20that%20activate%20the%20apoptosis.jpg?width=630&height=330&name=CAR%20molecules%20(light%20blue)%20bind%20to%20CD19%20molecules%20on%20a%20leukemia%20cell%20leading%20to%20segregation%20of%20granzyme%20vesicles%20(yellow)%20that%20activate%20the%20apoptosis.jpg)
CAR molecules bind to CD19 molecules on a leukaemia cell
‘We were giving it to patients who failed four, five, six, seven prior therapies – patients who were really at the end of the therapy menu, so these are very exciting results’ says Horak.
Tessa Therapeutics is not alone in trying to harness the power of allogeneic therapies. The number of allogeneic cell therapies for cancer in clinical trials grew globally by 30% between 2016 and 2021. By 2021, more than a quarter of all cell-based therapies being trialled for cancer were allogeneic, including 30% of such trials in the UK.
These efforts are paying off. In December 2022, an allogeneic T-cell therapy was given regulatory approval for the first time. Ebvallo, which is designed to treat a rare blood cancer, is the first off-the-shelf T-cell therapy to be given authorisation anywhere in the world.
‘There's an enormous amount of activity there and we're all tremendously excited by the potential,’ says Connolly, before quickly adding that we’re not there yet.
The world’s first off-the-shelf T-cell therapyTCAR-T therapies may have changed the way we treat some cancers, but a different type of T-cell therapy has won the allogeneic race. In December 2022, the dream of off-the-shelf T-cell therapies became a reality when Ebvallo was given marketing authorisation by the European Commission. The first allogeneic T-cell therapy to receive regulatory approval anywhere in the world, it is designed to treat a blood cancer called Epstein-Barr virus positive post-transplant lymphoproliferative disease (EBV+PTLD). This very rare condition can occur after an organ or bone marrow transplant when immunosuppression allows the Epstein-Barr virus to infect B-cells. Unlike CAR-T therapies, Ebvallo doesn’t involve engineering the T-cells to home in on the cancer. Instead, Ebvallo, from Atara Biotherapeutics, is made by mixing T-cells from a healthy donor with B-cells that have been infected with the Epstein Barr virus. This ‘trains’ the T-cells to recognise B-cells infected with the virus as ‘foreign’. Grown in the lab before being given to the patient, the T-cells then hunt down and kill the infected cells, helping control both the infection and the cancer. |
Hurdles to overcome
For all their potential, allogeneic CAR-T therapies still have their challenges. One of the biggest is graft-versus-host disease (GvHD), in which the donor cells recognise the cancer patient’s healthy tissue as foreign. ‘It’s a complicated manifestation of skin and gut and, eventually, lung – and it will kill you,’ explains Connolly. ‘It's a deadly disease.’
Strategies being used to sidestep GvHD include using gene-editing tools such as CRISPR, TALENs and new kid on the block, base editing, to delete the TCR, which is a receptor on the surface of T-cells that recognises foreign tissue. It was base editing that was used to modify the CAR T-cells in the world-first therapy given to Alyssa in 2022.
Another option, and one being employed by Tessa Therapeutics, is to use T-cells that have been generated in response to a virus. Experience from bone marrow transplants suggests that T-cells generated in this way can be infused into a patient without triggering GvHD.
Focus is also turning to other types of immune cells that are much less likely to cause an immune response, such as natural killer (NK) cells and gamma-delta T-cells. These cells can also be modified to enhance their therapeutic effect, including carrying a CAR into the patient’s body.
%20destroying%20a%20cancer%20cell.jpg?width=638&height=334&name=NK%20Cell%20(Natural%20Killer%20Cell)%20destroying%20a%20cancer%20cell.jpg)
Natural Killer cell destroying a cancer cell
The second major hurdle to overcome is rejection, when the cancer patient’s immune system attacks the infused cells. Rejection is not an issue for the current autologous therapies because the patient’s own immune cells can live on in the body, where they prevent the cancer from returning. In some cases, immune cells have been detected in the patient’s body more than a decade after they were administered. In contrast, off-the-shelf treatments can be rapidly eliminated by the patient, which reduces the effectiveness of the treatment.
Strategies to allow immune cells from allogeneic therapies to thrive in the patient’s body are now top of the agenda. One way is to hide the cells from the cancer patient’s immune system by deleting the T-cells’ MHC antigens – the proteins that signal the T-cells’ ‘foreignness’. Another option is putting inhibitory receptors on the surface of the T-cells to stop the patient’s cells from recognising them.
Treating other conditions
It's not all about cancer, though. Allogeneic cell therapies are also in development to treat a host of other conditions, from multiple sclerosis and strokes to osteoarthritis and diabetic kidney disease.
BlueRock Therapeutics, a subsidiary of Bayer, is working on off-the-shelf therapies for conditions as varied as Parkinson’s disease, age-related macular degeneration and heart failure.
Most of these therapies are based not on T-cells, but on induced pluripotent stem cells (iPSCs) – cells from the adult body that have been ‘wound back’ to an embryonic stem cell-like state. Such cells can then be instructed to turn into any cell type and, in theory, in unlimited numbers.
Here, the challenges are different to those faced by the developers of off-the-shelf CAR-T therapies. One is understanding the developmental biology, the pathways that need to be activated or blocked to turn an iPSC into the required cell type.
Knowledge from mice and fruit flies, favourite model organisms of developmental biologists, has to be extrapolated to the human body, all while bearing in mind that processes taking just weeks in mice take many months in humans.
For BlueRock, one of the biggest challenges is that it is spoilt for choice, according to Dr Mark Tomishima, its VP of platforms discovery. ‘The beauty of pluripotent stem cells is that we can make any cell in the adult body,’ says Tomishima, who previously worked at Memorial Sloan Kettering Cancer Center and took the foundational science, cells and IP with him when he moved to BlueRock.
Pluripotent stem cells on a microscopic background
‘One of the challenges we have is being focused. Part of my job is being able to edit the genome of those cells to weaponise them. This is a wonderful luxury and there are probably tens of thousands of different kinds of medicines that you might imagine we could create.
‘I hope that as we move forward, we are going to cure a number of diseases which will unlock resources and let us be defocused. But we have to keep that laser-sharp focus at the beginning.’
In the case of BlueRock’s lead pipeline candidate, human pluriopotent stem cells have been used to generate dopaminergic neurons, the brain cells that are lost in Parkinson’s disease, leading to tremors, stiffness and a gradual slowing of the body.
One of the first allogeneic stem cell therapies for Parkinson’s, bemdaneprocel (BRT-DA01) has been injected into the brains of 12 patients from the US and Canada. In preclinical studies, the injected dopamine-producing cells eased movement symptoms caused by a lack of endogenous dopamine and the first results from the phase 1 trial are expected in the second half of 2023.
So, where could the technology take us in 20 years’ time? Tomishima believes synthetic biology could be transformative.‘ In collaboration with Senti Bio, we are trying to encode genetic logic gates into our cells that would endow them with new behaviours,’ he says. ‘One intuitive example is a cell that could sense its environment and respond in a differential way.
‘Let’s say that inflammation is a problem in a patient. Right now, if you take a drug for inflammation, it’s typically going to flood throughout your whole body.
‘What we are imagining is a situation where our cells are in the patient and then go specifically to the region that’s damaged, sense that they’re in that region and then respond with the appropriate cargo. That could be a cytokine, it could be an antibody, it could really be anything you can think of, anything we can weaponise our cells with.’
iPSCs, with their potential to grow in limitless numbers, will also be at the forefront of allogeneic CAR-T therapies, says Connolly. For a cancer patient, this will mean same-day treatment with high quality cells that have been engineered to live on in the body. The patient may then come back to be treated with further courses of CAR-T therapy, reducing the need for chemotherapy and radiotherapy and their associated side-effects.
‘The advances we’ve made here in the past 15 years are bigger than the past 5,000 years of cancer research,’ says Connolly. ‘Our ability to see and get complete responses in some extremely difficult tumours – we haven't seen anything like that since the advent of surgery in ancient Egypt.
‘And what we're working on is just the beginning. Trying to make this something that makes all cancers curable diseases is really the goal and we'll get there for sure.’
Dawn of allogeneic therapies
Anna Mudge, Mewburn Ellis Senior Associate and Patent Attorney, comments:
The dawn of allogeneic cell therapies is truly exciting and holds potential for treating all kinds of diseases. The availability of ‘off the shelf’ cells will dramatically increase patient access to crucial therapies around the world, whilst reducing unwanted immune responses and the need for harmful immunosuppression. Patients and healthcare systems will also enjoy reduced treatment costs, benefiting from cell production on a commercial scale rather than for separate individuals. Challenges remain, as with any emerging technology, but we expect these to fall away as the field matures. We look forward to seeing how the technology develops and are excited to work with the innovators in this area.
Written by Fiona MacRae
Anna is a Partner and Patent Attorney at Mewburn Ellis, specialising in life sciences. Her practice spans IP strategy, drafting, prosecution, and oppositions, with expertise in managing complex, high-value patent portfolios and successful EPO oppositions. She leads the firm’s Advanced Therapeutics team, advising on cell and gene therapies, nucleic acid technologies, and immunotherapies. Anna works with biotech start-ups, universities, and multinational pharmaceutical companies across the globe, and is a regular visitor to the US East Coast. She holds a first-class BSc in Molecular Biology and Biochemistry and a PhD in Molecular Cell Biology from Durham University.
Email: anna.mudge@mewburn.com


