Cardiovascular disease is estimated to account for 45% of deaths in Europe each year. More specifically, in the UK the heart transplant waiting list has increased by over 140% since 2010. There is a clear need to develop alternative solutions to human-human heart transplants, which would allow patients with heart failure to live normal lives. In this article we look at some of the recent advances in this field.
A total artificial heart (TAH) is one such alternative, replacing the entire function of the native heart. Candidate patients are those with bi-ventricular heart failure, with TAH devices currently used as a bridge to transplant rather than as a permanent solution. The end-goal of these devices is to provide a permanent replacement to the native heart, allowing recipients to live healthy, happy, and active lives.
Pumping blood around the human body provides an array of challenges as evidenced by the various attempts to create a working TAH in previous decades. However, recent developments from different groups developing TAH devices mean we should be optimistic that a more effective and practical solution could soon be available.
History and Challenges
The Jarvik 7 was the first total artificial heart that functioned beyond keeping a patient alive for a matter of hours during surgery. The first successful transplantation of the Jarvik 7 was in 1982, with the patient living for 112 days. Through various iterations and improvements, the Jarvik 7 is now the only total artificial heart to have FDA approval as a bridge to transplant solution (since 2004) and is currently marketed as the SynCardia temporary total artificial heart. Each native ventricle is replaced by an air chamber and a blood chamber. The pumping action works by the pressure in the air chamber increasing, contracting the corresponding blood chamber, and forcing blood into the artery.
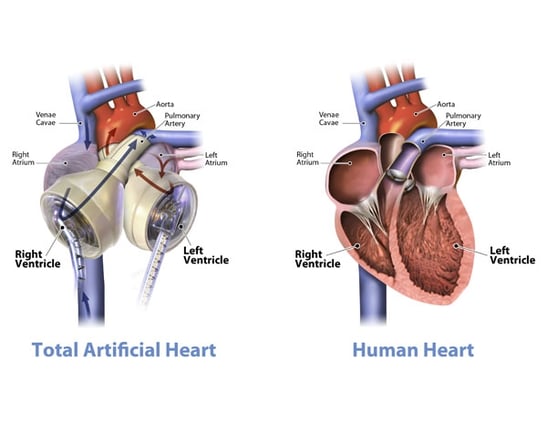 |
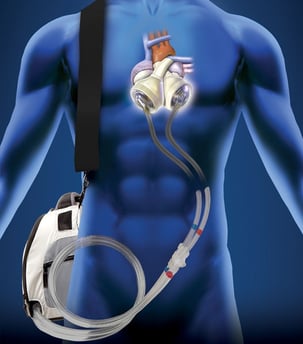 |
| SynCardia TAH | |
A wide variety of problems face groups working on TAH devices. The risk of infection is very high, particularly if connection tubes or power supplies pass through the skin. Furthermore, thrombus formation is common on blood-contacting artificial surfaces and areas of the TAH that are not completely washed out during pumping. Damage to blood cells caused by the TAH pumping action is yet another issue, as well as the complications of balancing the systemic and pulmonary flows. This is before even considering the efficacy of the pumping action and longevity of the device.
The quality of life for TAH recipients is also very limited, with patients having to carry an external rechargeable battery pack and other equipment, which are bulky and noisy. A number of technological developments aim to address these issues.
Recent Developments
There are several active groups developing TAH devices, each with their own advances aiming to overcome the challenges laid out above.
Carmat is a French company which has developed the Aeson TAH, a pulsatile bi-ventricular pump which uses biological valves, as well as a bovine pericardial tissue layer on the blood contacting surfaces of the artificial ventricles. This aims to prevent thrombus formation on the artificial surfaces. This is a major concern for TAH devices as if a thrombus does form and is then dislodged, it has the potential to narrow or even block the blood vessels. Carmat has obtained a CE mark but has yet to receive FDA approval.
Scandinavian Realheart have developed a TAH designed to mimic the native heart as closely as possible to avoid common TAH-related complications such as stroke, bleeding, and anaemia. Through research on the pumping action of the native heart, they have determined that the native heart acts analogous to a piston pump. As well as the piston action, the Realheart is the only TAH which includes atria as well as ventricles to generate a physiological blood flow pattern, mimicking the circulation of the native heart within the device. The Realheart TAH is currently in the animal trial phase of development.
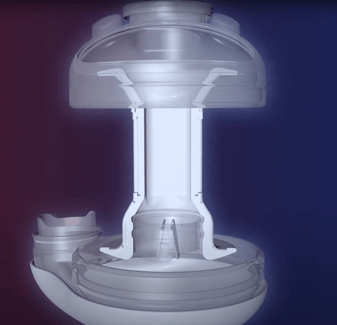
Scandinavian Realheart TAH
BiVacor have taken the opposite approach, dispensing with the requirement to mirror the pulsatile action of the native heart, to overcome the challenges that previous devices have faced. They have developed a centrifugal TAH, which in normal operation delivers non-pulsatile flow of blood around the body. This allows the device to have fewer moving parts, which together with contactless bearing technology, reduces mechanical wear of components, increasing the longevity and efficiency of the device. Earlier this year, BiVacor received $18m in funding for initial human trials.
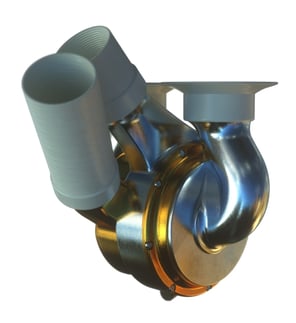
BiVacor TAH
As well as these companies, many universities are launching and growing teams to compete in the Heart Hackathon, a new student competition to design a TAH. The inaugural grand final was held at the 2023 International Society for Mechanical Circulatory Support Conference in Texas, featuring 8 teams from across the world. TeamBath Heart from the University of Bath won first-prize for their design.
Barriers remain to develop the permanent total artificial heart into a true human-human heart transplant alternative, not least of which is overcoming all of the aforementioned problems in a single device. Commercial challenges also exist, with SynCardia declaring bankruptcy in 2016 before being acquired by an investment firm and AbioMed abandoning their FDA approved AbioCor TAH in 2016 as it was deemed commercially unviable.
Conclusion
The benefits these devices could have for patients experiencing heart diseases is clear, providing first a way to survive until a human heart is available and, in the future, a long-term solution to the shortage of donor hearts that has been a growing problem for decades.
It is exciting to see different groups using modern technology to solve decades old problems. It will be interesting to see how these devices perform in clinical trials and beyond.
Andrew is a Partner and Patent Attorney at Mewburn Ellis. He deals with drafting and prosecuting patent applications at the EPO and UKIPO, as well as global patent portfolio management, Freedom-to-Operate (FTO) work and advising on global patent filing strategies. Andrew works in the engineering and electronics fields, with a focus on medical device technologies. He has spent time working as a patent attorney in Singapore, where he specialised in providing advice on obtaining patent protection throughout South-East Asia, China and the Indian sub-continent. Andrew has also worked in Canada, developing an expertise in obtaining patent protection in North America.
Email: andrew.mears@mewburn.com

.png)
-Dec-29-2025-09-11-25-2361-AM.png)