In our previous blog post 'Naming pharmaceuticals: considerations for choosing, clearing and registering pharmaceutical brand names', we looked at considerations for choosing the name of a pharmaceutical product, but there are many other aspects of branding which might warrant protection. Most brand owners will have considered whether there are additional word or logo trade marks they should protect, but some might overlook the less traditional types of trade marks.
What are non-traditional trade marks?
This term typically includes any type of trade mark beyond a word mark, logo mark, or logo containing words. In the UK and EU, it is possible to protect shapes, colours, sounds, motion marks, position marks, holograms and pattern marks, providing they are capable of (a) being represented in the register in a manner which enables people to determine the clear and precise subject matter of the protection afforded, and (b) distinguishing goods or services of one undertaking from those of other undertakings.
Applications must comply with the criteria established in Sieckmann, namely that the representation of the mark must be clear, precise, self-contained, easily accessible, intelligible, durable and objective.
In reality, some types of marks, such as smell and taste marks, are currently unacceptable as they simply cannot be filed in a way which complies with all the above requirements.
In this blog post, we will look at one specific type of non-traditional trade mark in the UK and EU, namely shape marks, examining their relevance in the pharmaceutical sector and the particular challenges faced.
What shapes can be registered?
A distinctive shape to a product or its packaging can add considerable value to a brand. If it is quite unique, consumers are likely to come to see that shape as indicating brand origin, a role traditionally performed by the brand name and logo. Of course, the more iconic a shape becomes, the more likely it is that it will be copied, causing confusion. A case of mistaken identity in the pharmaceutical sector can have very serious consequences. For that reason, brand owners might be interested in registering particular shapes that are key to their branding, such as blister packs, tablets and bottles.
Novartis has protected both the packaging of their cancer drug GLIVEC and the shape of the tablets themselves (with the word imprinted onto the surface of the tablet).
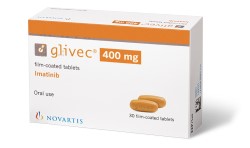 |
 |
| EUTM Registration No. 13244629 | EUTM Registration No. 13228937 |
It is also common to protect the shape of products designed to deliver medicine, for example, Luye Pharma’s EUTM Registration No. 12927935 covering “Medical and veterinary preparations and articles, Namely rod-shaped medicine implants” and “Medical and veterinary apparatus and instruments, Namely cannula devices for holding and administering rod-shaped medicine implants”; and AstraZeneca’s EUTM Registration No. 5695341 covering “Pharmaceutical preparations” and “Medical and surgical apparatus and instruments; inhalers”.
 |
 |
| EUTM Registration No. 12927935 | EUTM Registration No. 5695341 |
There are equivalent comparable UK registrations for all of these EUTMs.
Challenge 1 – Distinctiveness
Shapes are often not considered to be inherently distinctive, as consumers will typically see them as decorative or functional, rather than as indicating the origin of the product. This means that even if you have presented your shape in a clear, precise way, objections may be raised on the grounds that the mark is devoid of distinctive character.
Abtei Pharma Vertriebs’ application to register a pill shape under EUTM Application No. 6093141 was refused on the grounds that the disc shape of the pill is commonplace and the other main feature, the grooves, are purely functional, facilitating the breaking of the pill into two or more pieces.
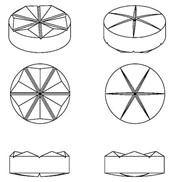
EUTM Application No. 6093141
Gathering and filing evidence of extensive use and arguing that the mark has acquired distinctive character through use may help to overcome the objection, but this can be a costly exercise, and if the application is an EU trade mark application, you will need to prove acquired distinctive character across the whole of the EU, which is a significant burden.
One way round this problem can be to include additional distinctive elements. Pfizer’s EU and comparable UK trade mark registrations for the shape of their VIAGRA “little blue pill” include the Pfizer name as it appears on the tablet and therefore no evidence of acquired distinctive character was required for registration.
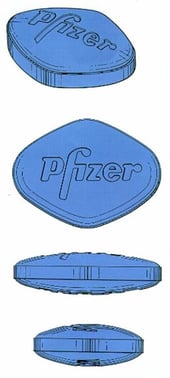
EUTM Registration No. 1909472
On the other hand, an application to register the mark without the brand name was refused.
It will be important to bear in mind that the additional words will also be taken into account when assessing whether there is infringement in the future. If the contested sign does not contain an element similar to the distinctive word contained in the registered shape mark, there may be no risk of confusion on the part of consumers.
Challenge 2 – Specific shape grounds for objection
UK and EU trade mark law also include some specific provisions relating to shape marks. Marks are precluded from registration if they consist exclusively of (a) the shape or another characteristic that results from the nature of the goods themselves; (b) the shape or another characteristic of the goods that is necessary to obtain a technical result; or (c) the shape or another characteristic of the goods that gives substantial value to the goods.
Pharmaceutical shapes may be particularly vulnerable to objections based on technical function. In October 2012, Novartis applied to register the following mark:

EUTM Registration No. 11293362
Registration was granted in March 2013 in respect of “Pharmaceutical preparations for the treatment of dementia of the Alzheimer's type”. The registration corresponded to the product Exelon, administered by transdermal patch, which enjoyed patent protection until July 2012. The active pharmacological substance was rivastigmine.
Shortly after registration, SK Chemicals GmbH, which manufactures generic medicinal products, including transdermal patches for the administration of rivastigmine, applied to cancel the registration.
The EUIPO concluded that all the essential elements of the mark, namely the square shape of the patch; the overlapping protective plastic layer represented by the white stripe in the background of the mark; the circular area in the centre; and the arrangement of knobs around the central circular area, were all designed to perform a technical function.
“The square shape is motivated by manufacturing concerns of efficiency, material loss, ease of packaging and storage. The overlapping plastic layer allows easy application of the patch on the body and prevents the patch from being exposed prior to use... The circular area in the centre… is the part of the patch which contains the medicinal compound with which patients are treated… a circular shape is very common in transdermal patches and allows the patch to adapt to body movements thus ensuring a better affixation to the skin than other shapes would. In that sense it performs a function. The use of knobs creates space between patches during transport. This space reduces the loss and exposure of the medicinal substance which occurs via the ‘cold flow’ effect i.e. when the patch sticks to its packaging.”
The registration was therefore declared invalid, a decision which was upheld on appeal. Novartis still owns trade mark registrations for variations of the mark with additional elements.
Can shape marks be enforced?
The circumstances in which it will be appropriate to try and enforce a shape mark are likely to be more limited compared to enforcement of more traditional trade marks. In addition, in view of their weaker inherent distinctiveness, they may have a more limited scope of protection, and you may be required to submit supporting evidence. However, if validly registered, shape marks can be enforced effectively, just like any other type of trade mark.
By way of example, in January 2019, the EUIPO Board of Appeal declared EUTM Registration No. 9849191 for “inhalers” and “inhalation products used for the treatment of asthma and chronic pulmonary disease” invalid on the grounds of a likelihood of confusion with Glaxo Group’s earlier Hungarian Registration No. 173643, which covers “inhalers”.
 |
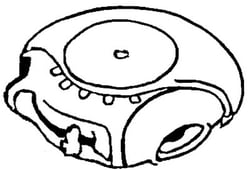 |
| EUTM Registration No. 9849191 | Hungarian Registration No. 173643 |
In our next blog post in this series, we will look at some of the other types of non-traditional trade marks relevant to the pharmaceutical sector.
Our Forward: Feature 'Pharma brands: What's in a name?' explores the exacting creative journey required to pin down a new drug name that will both catch attention and clear regulatory hurdles. Read the full article.
Rebecca is a Partner and Chartered Trade Mark Attorney at Mewburn Ellis. She handles all aspects of trade mark work, with a particular focus on managing large trade mark portfolios, devising international filing and enforcement strategies, and negotiating settlements in trade mark disputes. Rebecca has extensive experience of trade mark opposition, revocation and invalidity proceedings before the UK Intellectual Property Office (UKIPO), including very complex evidence based cases. Rebecca also has a strong track record in overcoming objections raised to trade mark applications.
Email: rebecca.anderson@mewburn.com


