As we enter 2024, we reflect on the lessons we have learned in 2023. The trade mark landscape has continued to evolve, and pharmaceutical trade marks are no exception. In this article, we take a quick look at some interesting decisions issued by the EU General Court concerning pharmaceutical trade marks and highlight key learning points for the future.
Pascoe pharmazeutische Präparate v EUIPO – Novartis
[General Court, Case T-435/22]
Context and Background: In this case, Pascoe pharmazeutische Präparate GmbH contested the decision of the European Union Intellectual Property Office (EUIPO) allowing the registration of Novartis Pharma AG’s trade mark "PASCELMO". Pascoe was of the opinion that "PASCELMO" was too similar to Pascoe’s own trade mark, "PASCOE", and could potentially cause confusion in the market.
Central Issues: The legal question centred around the concept of 'likelihood of confusion' – essentially, whether the two trade marks were so similar that people might mistakenly believe they come from the same company. Likelihood of confusion is assessed based on the overall perception of the marks by the relevant public, taking into account the similarity of the goods or services protected under the marks and the similarity of the signs. In this case, the goods were identical, and Pascoe only challenged the EUIPO’s assessment of the sign and their overall assessment of the likelihood of confusion.
Court’s Analysis and Decision:
1. Looking at the Trade Marks: The Court re-examined the visual and phonetic impression that the trade marks "PASCELMO" and "PASCOE" made. They noted that although both marks start similarly, the relevant public would perceive the rest of the marks as visually and aurally different. They considered that the marks only had a low degree of visual and aural similarity.
2. Overall assessment - understanding the relevant public: The Court considered the overall impression of the marks and if a likelihood of confusion, after considering all factors, existed. They confirmed previous case law, which states that in the pharmaceutical sector, both healthcare professionals and everyday consumers tend to pay more attention to product names, given the impact of the products on health.
3. Final Judgment: Considering these factors, the Court decided that the two trade marks were different enough not to cause confusion, even if they were registered for identical products. Since there was no likelihood of confusion, Novartis’ "PASCELMO" trade mark could proceed to registration.
Implications and Takeaway for the Pharmaceutical Industry: This case is a reminder that there are certain nuances that are applicable to pharmaceuticals that may not be generally applicable. In the pharmaceutical industry, customer attention is generally high because the impact on health is higher. This means that smaller differences in trade marks can be enough to prevent a likelihood of confusion, even if the products are identical. This may not have been the case in a different industry, where customer attention is merely average or even below average.
Hecht Pharma GmbH v EUIPO – Gufic BioSciences Ltd
Context and Background: Hecht Pharma GmbH challenged the decision of the EUIPO regarding the trade mark "GUFIC," which was contested for lack of genuine use. Gufic BioSciences Ltd, the trade mark owner, defended their registration, presenting evidence of its use for “medicine”. The key issue was whether the "GUFIC" trade mark was genuinely used in line with the EU trade mark regulation (EUTMR).
Central Issues: The central legal question revolved around the interpretation of 'genuine use' of a trade mark within the EU. ‘Genuine use’ encompasses use in accordance with the essential function of a trade mark, namely to guarantee the identity of the origin of the goods or services for which it is registered, in order to create or preserve an outlet for these goods. It requires that the use of the mark is public and outward.
In this case, the focus was on whether the products marked with "GUFIC" were genuinely used in their registered form for “medicine”.
Court’s Analysis and Decision:
1. Assessment of the genuine use of the trade mark: The Court analysed the genuine use of the trade mark, especially with regard to the public and outward use of the mark.
Gufic BioSciences presented several invoices addressed to German pharmacies and an affidavit from an intermediary. The Board of Appeal stated that these invoices constituted public and external use of the contested trade mark and that, with all things considered, Gufic BioSciences had succeeded in demonstrating genuine use for “medicine”.
Hecht argued that there was insufficient public and outward use of the mark, and that Gufic BioSciences’ distribution model was illegal. In order to show genuine use, Gufic BioSciences would have needed to show sales from pharmacies to end customers. Furthermore, Gufic BioSciences was unable to show advertising under the “GUFIC” mark.
The Court rejected Hecht’s complaint, stating that the EUIPO is not authorized to comment on the compliance of distribution systems with national law. Furthermore, illegal distribution models don’t preclude ‘genuine use’ of a trade mark under the EUTMR. It follows from this that the invoices Gufic BioSciences presented can constitute evidence of public and outward use and that this is not equivalent to sales to end customers.
The Court concluded additionally that the lack of advertisement of the goods doesn’t exclude a finding of genuine use, especially when advertisement for the goods is forbidden under national law, as it was in the case at hand.
2. Trade mark Use in Registered Form:

Hecht argued that the addition of the elements “H 15” on the products leads to a different overall impression than “GUFIC” and therefore didn’t constitute genuine use. Furthermore, the addition of “Made in India by:” gave the impression that “GUFIC” was a company name.
Firstly, the Court clarified that a trade name, while not typically intended to distinguish goods or services, can be used as a trade mark if it’s used on goods or packaging. Further to this, consumers are used to being confronted with multiple trade marks in the pharmaceutical sector, namely both the product and manufacturer’s marks.
The Court followed from this that despite variations in presentation (H 15, yellow half circle), the core element of the trade mark “GUFIC” remained intact and distinctive, thus fulfilling the function of a trade mark and the requirements for use in the registered form.
3. Use for Registered Goods – “Medicine”: Lastly, the Court deliberated on whether the Board of Appeal was correct in its assessment that the products bearing the "GUFIC" trade mark qualified as “medicine” (for which the trade mark was registered), since the products were classified as “ayurvedic medicine” under EU law.
The Court based its decision on the perception of the goods by the relevant public. In previous cases, the Court had decided that the sale of goods in pharmacies did not necessarily qualify goods as “medicine”. However, Gufic BioSciences’ products were sold in pharmacies only upon presentation of a prescription from a doctor. This would influence the relevant public to perceive the goods as “medicine”. The goods also contained a medical warning sheet. From this, the Court concluded that the Board of Appeal hadn’t erred in basing their decision on the assumption that the goods were “medicine”.
Implications for the Pharmaceutical Industry: This judgment provides useful guidance on the genuine use of pharmaceutical trade marks. When considering pharmaceutical products or consumer healthcare products, we often have to look beyond the scope of trade mark law and consider if there are other regulatory factors at play, such as national laws regulating advertising. While under normal circumstances advertising material would usually be included as a portion of proof of use, this isn’t necessarily always possible for pharmaceuticals.
This case also reminds us that the consumer perception when considering pharmaceutical trade marks is key and that it may be different than when considering every day goods. Consumers expect to be confronted with both the manufacturer’s mark and the product trade mark.
This decision was appealed to the Court of Justice of the European Union in case number C-142/23 P. The Court of Justice will need to decide if it will hear the appeal before the decision is final.
Glaxo Group Ltd v EUIPO
[General Court, Case T-477/21]
Context and Background: The General Court addressed a dispute between Glaxo Group Ltd and the EUIPO, with Cipla Europe NV as an intervening party. The case centred around whether a three-dimensional EU trade mark representing an inhaler shape, originally registered by Glaxo Group Ltd, should be declared invalid:
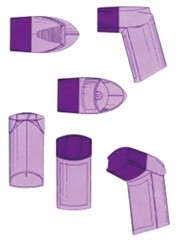
Central Issues: While the main focus of this case was procedural issues regarding the Board of Appeal’s failure to state reasons, the General Court also confirmed some case law regarding the assessment of the distinctive character of a trade mark and market behaviours in the pharmaceutical industry.
Court’s Analysis and Decision
1. The decision of the Board of Appeal: The Court identified and analysed contradictions in the reasoning of the Board of Appeal. The Court noted inconsistencies, particularly in the assessment of the trade mark's distinctive character at the time of its registration in 2001. The Board of Appeal had correctly determined that the relevant time period for the assessment of distinctiveness was the registration of the mark in 2001 and noted that, in their challenge, Cipla had failed to provide sufficient evidence in this regard.
However, the Board of Appeal went on to determine that the mark lacked distinctive character as the colour would be seen as being descriptive for the medicine contained therein. The Board of Appeal based this conclusion on undated evidence provided by Cipla and principles set forth in the decision of the General Court The shade of the colour purple (GC, judgement of 9 September 2020, T-187/19). The shade of the colour purple referenced the market behaviour in 2015, so a good fourteen years after the relevant time in the case at hand.
2. Annulment of Decision: Due to these contradictions and the failure to provide clear, consistent reasoning, the General Court annulled the decision of the EUIPO’s Board of Appeal, and Glaxo’s registration remained on the registry.
Implications for the Pharmaceutical Industry: This judgement emphasizes the complexities of assessing the distinctiveness of trade marks in the past. Especially in fast-paced sectors such as the pharmaceutical sector, the market changes rapidly. The colour of an inhaler could be distinctive today and descriptive in a few years.
When launching an invalidity challenge against a trade mark that has been registered for some time, it is essential you can provide credible evidence that the mark was not distinctive at the time of filing. Otherwise, it should be considered if you could revoke the mark for becoming generic through the acts or inactivity of the proprietor.
On the other hand, trade mark owners need to carefully review the manner in which they use their trade marks periodically to ensure they are used as trade marks, not generically. They should also keep an eye on third party use of the same and similar marks and take action to prevent any use which may cause the mark or its features to be deemed generic.
Further analysis of this decision will follow in a separate blog post.
Novartis v EUIPO – AstraZeneca (BRETZERV / BRETZERI)
[General Court, Case T-175/22]
Context and Background: Novartis, the owner of three registered EU trade marks (BREZILIZER, BREEZHALER, and ONBREZ, protected for, inter alia, “pharmaceutical preparations”) filed an application for invalidity against AstraZeneca’s registration for BREZTRI (registered for “pharmaceutical preparations and substances”). The Cancellation Division and Board of Appeal both rejected the application due to lack of similarity of marks.
Central Issues: Novartis had argued that the general public doesn’t pay the same high degree of attention as medical professionals when selecting medicines, owing to a lack of medical knowledge. The Court disagreed. As such, the Board of Appeal had not erred in finding that consumers will pay the same elevated level of attention toward the marks, regardless of if they are consumers or medical professionals.
We have analysed this decision in more depth here.
This blog was originally written by Emily Sullivan.
Jan is a member of the trade mark team, with vast experience in all trade mark related matters from general strategic advice, clearances, filing and prosecution to enforcement of trade marks in opposition and court proceedings. His work includes negotiation of trade mark related agreements including out of court settlements, and managing Border Seizure applications. He also has experience in design and copyright matters.
Email: Jan.Rether@mewburn.com


